Conformation of polymers
Conformation is the position that each atom of the polymer takes on at a certain given time. In particular the position of atoms on adjacent carbons along the polymer chain, also called backbone, influence what positions that are preferred. The more electronegative and bulky atoms or side groups the more they avoid their proximity. In Figure 4 this is illustrated for a low molecular organic compound.
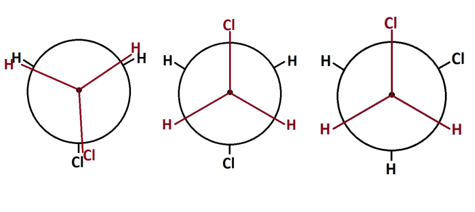
Figure 4. Newman projection of 1,2 dichloroethane molecule in different conformations
© Anders Persson, University of Borås
The likelihood of taking on the staggered position to the left in Figure 4 drops since a significant energy barrier must be overcome in order to rotate a whole revolution around the two covalently bonded carbons of the backbone. Transferred to a polymer this would give long ranging effects on the preferred overall shape of the whole polymer chain. If a polymer is free to find its preferred conformation it will try to reach its most disordered state. This is governed by the Second law of thermodynamics, which states that nature always moves towards higher entropy. Entropy in polymers can be interpreted as degree of disorder and is one of the major factors that control the behaviour of polymers.
The polymers are constantly moving around. This is called Brownian motion. The higher the temperature the more vigorously the movement becomes. It is just like gas pressure that raises with temperature since the gas molecules increase their velocity with temperature.





