Aromatic hydrocarbons
-
Introduction
Benzine
Seems to be part of the unsaturated hydrocarbons (double bonds) but they have so different properties that they form their own group.
Often have strong smell which has given them their names. Their nomenclature is based on a number of base structures: benzene, naphthalene, anthracene etc.
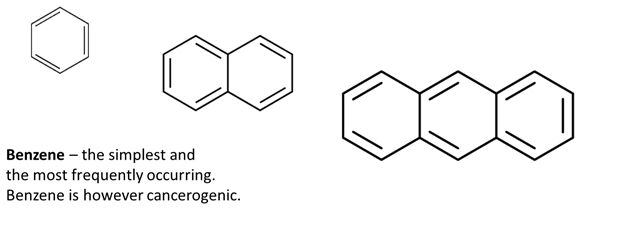
© University of Borås
© University of Borås
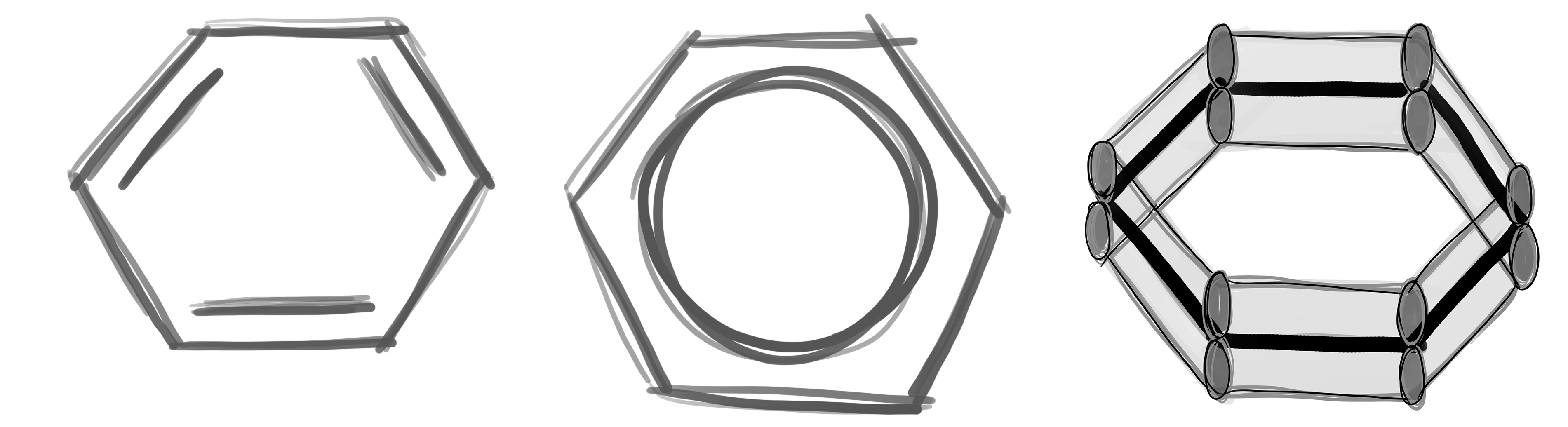
Numbering carbon atoms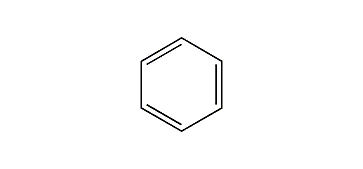
© University of Borås
- Benzene as a substituent = phenyl.
- The benzene ring consists of 6 carbon atoms and three double bonds.
- The double bonds are not locked in position so all six bonds in the ring are.
- Identical (this is called aromaticity – not only for carbon).
- This yields a stable molecule.
© University of Borås
Numbering of the carbon atoms in aromatic systems:
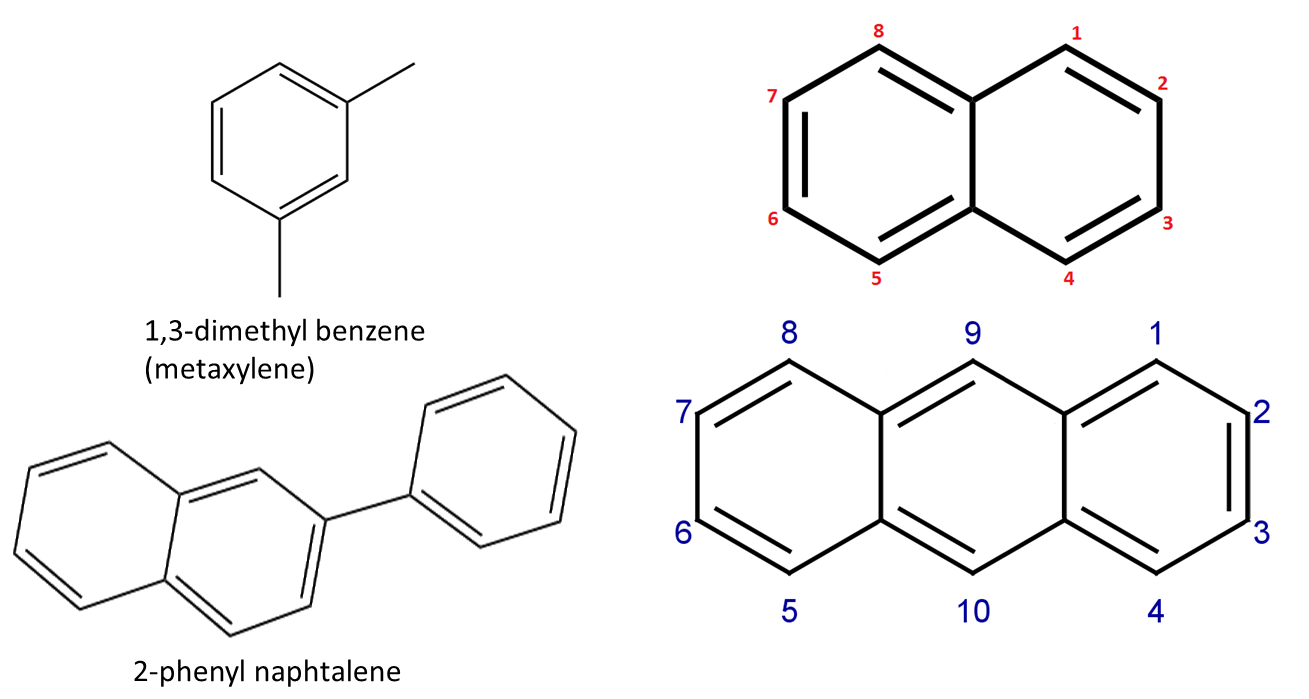
© University of Borås
-
Project
Contact
This resource was developed as part of an Erasmus+ project, funded with support from the European Commission under grant agreement 2016-1-SE01-KA203-22064.
The project was a collaboration between:
- The University of Borås, Sweden
- The University of the Highlands and Islands, Scotland
- The University of Alcalá, Spain
- Digital Connections, Scotland
This resource has been released under Creative Commons license CC-BY-SA 4.0.
Disclaimer
If you would like more information on this resource please contact:
- Academic content – The University of Boras (www.hb.se)
- Technical resource development – The University of the Highlands and Islands Educational Development Unit - EDU (edu@uhi.ac.uk)
Except where otherwise noted, this website is licensed under Creative Commons license CC-BY-SA 4.0. All images used under permission remain the copyright of the license holder.
Download a copy of this resource in PDF format.
You can also print individual pages by printing directly from the browser.
-





