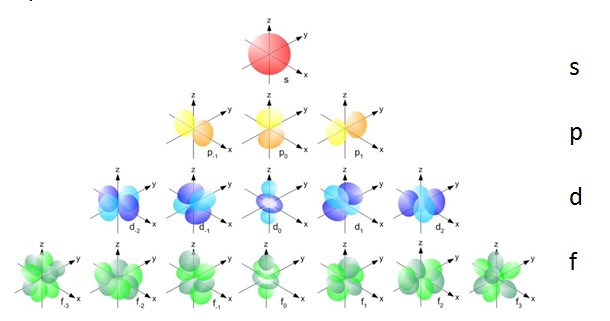
Orbitals
- A more advanced model than the electron shell model.
- Electrons are assigned to atomic orbitals that occupy different regions of space. The orbital is a theoretical volume where the electron exists 95 % of the time.
- The base orbitals are s, p and d.
Atomic Orbitals:
In every electron shell: 1 s-orbital
From, and including, shell 2: 3 p-orbitals
From, and including, shell 3: 5 d-orbitals
Max 2 electrons in each orbital!!
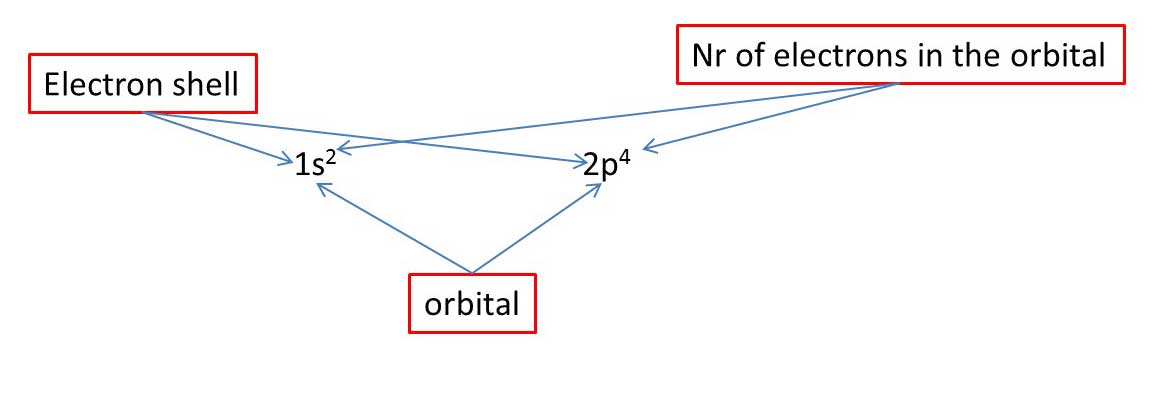
© University of Borås
How to fill the orbitals with electrons
When all have one electron we start to fill the orbitals with the second electron.
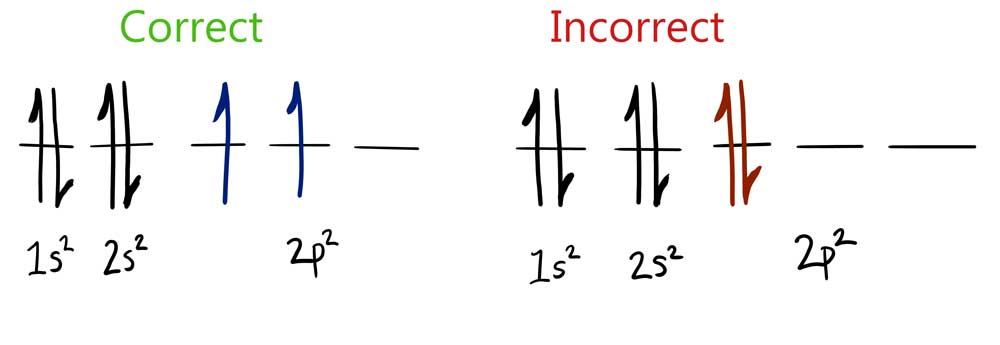
© University of Borås
Aufbau principle: The electron is always positioned in the orbital with the lowest energy
Pauli principle: There can be maximum 2 electrons in each orbital. The electrons have different spinn.
Hund’s rule: Orbitals with the same energy first get 1 electron each.