Atoms
Atoms: electrically neutral
Anion: negatively charged (addition of electrons)
Cation: positively charged (removal of electrons)
Isotope: atoms with equal numbers of protons and electrons but different numbers of neutrons.
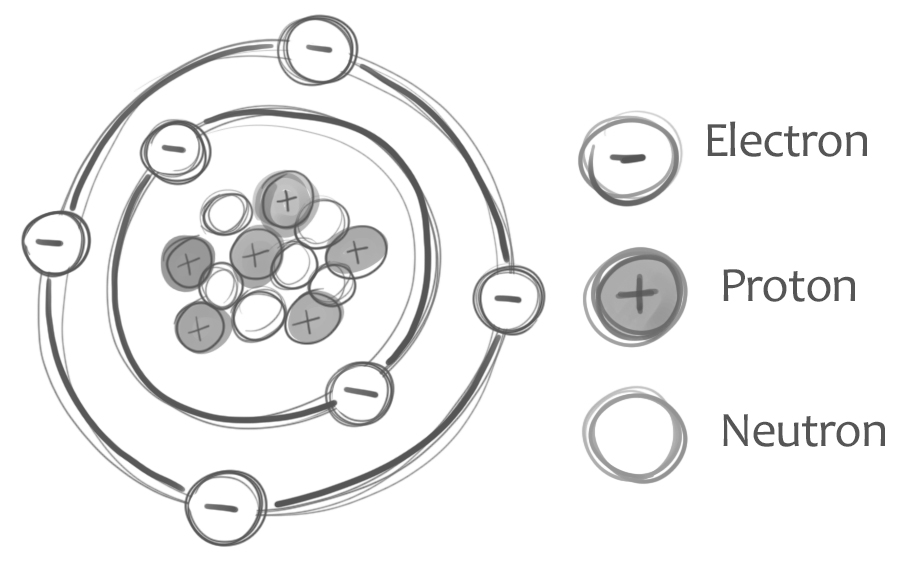
© University of the Highlands & Islands
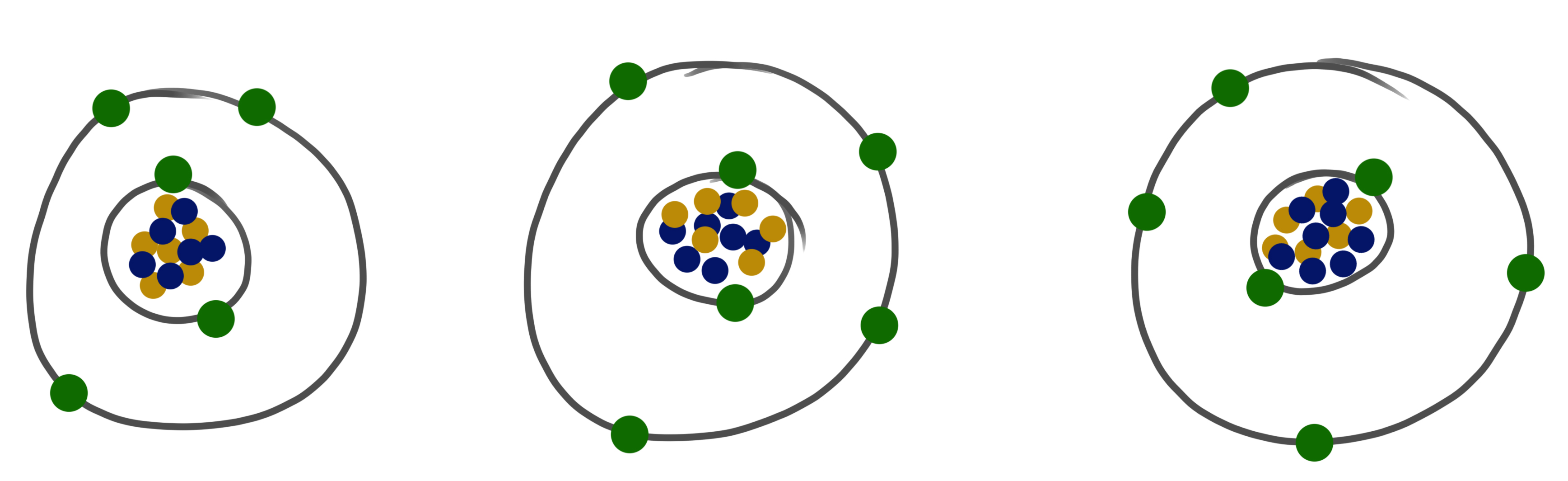
© University of the Highlands & Islands
Carbon atom: Normally 6 protons and 6 neutrons in its core (atom number 12, 12C).
- approx. 1% have an extra neutron (13C)
- an even smaller amount have two extra neutrons (14C)
- 14C is unstable, radioactive and decay with a half-life of 5730 years.





