Covalent bonding
The carbon atom: 4 valence electrons
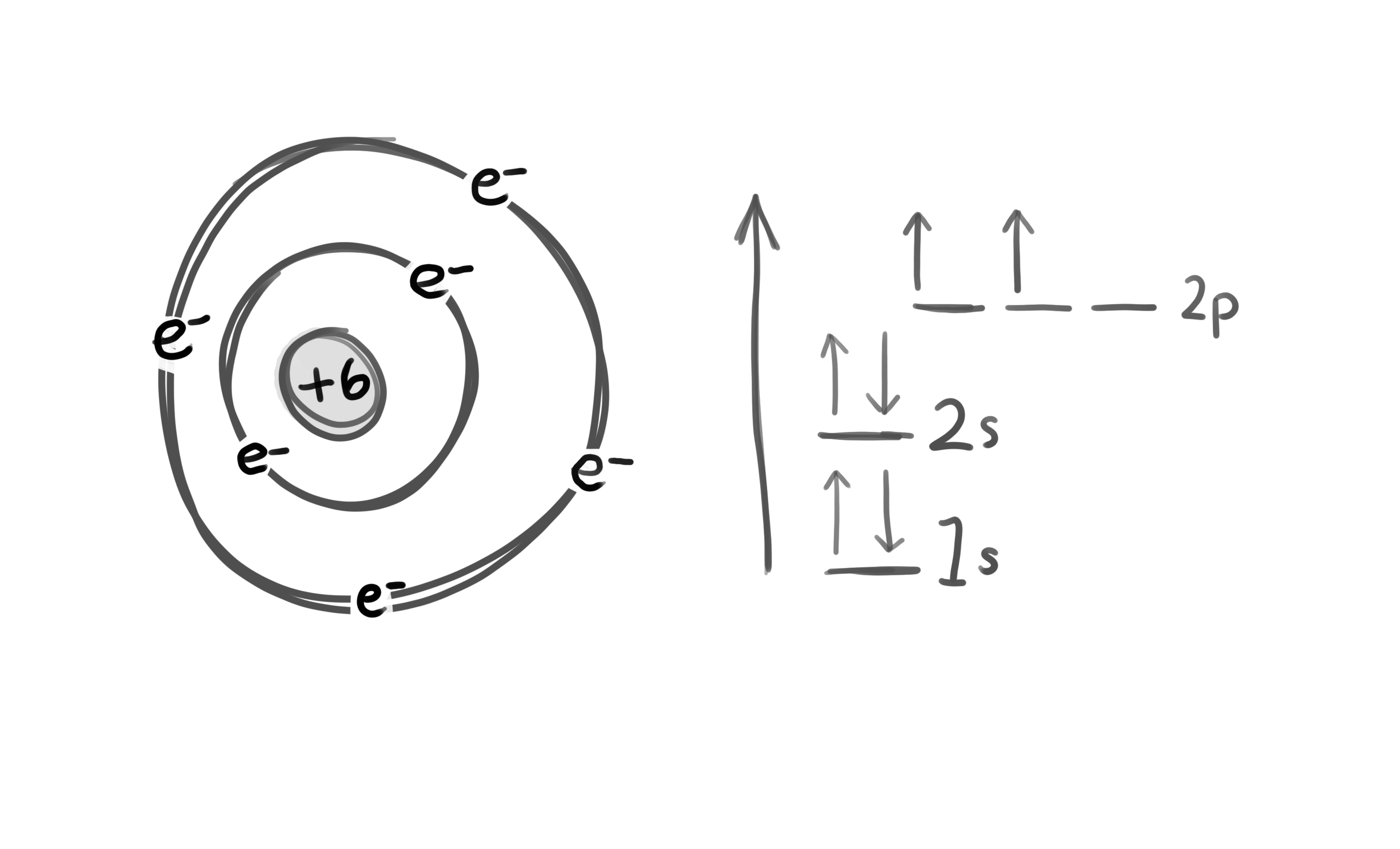
© University of Borås
This should result in 2 bonds with certain character and 2 bonds with another type of character (s- and p- orbitals)
However, carbon can form 4 identical bonds!!! How? The solution is hybridization!!





