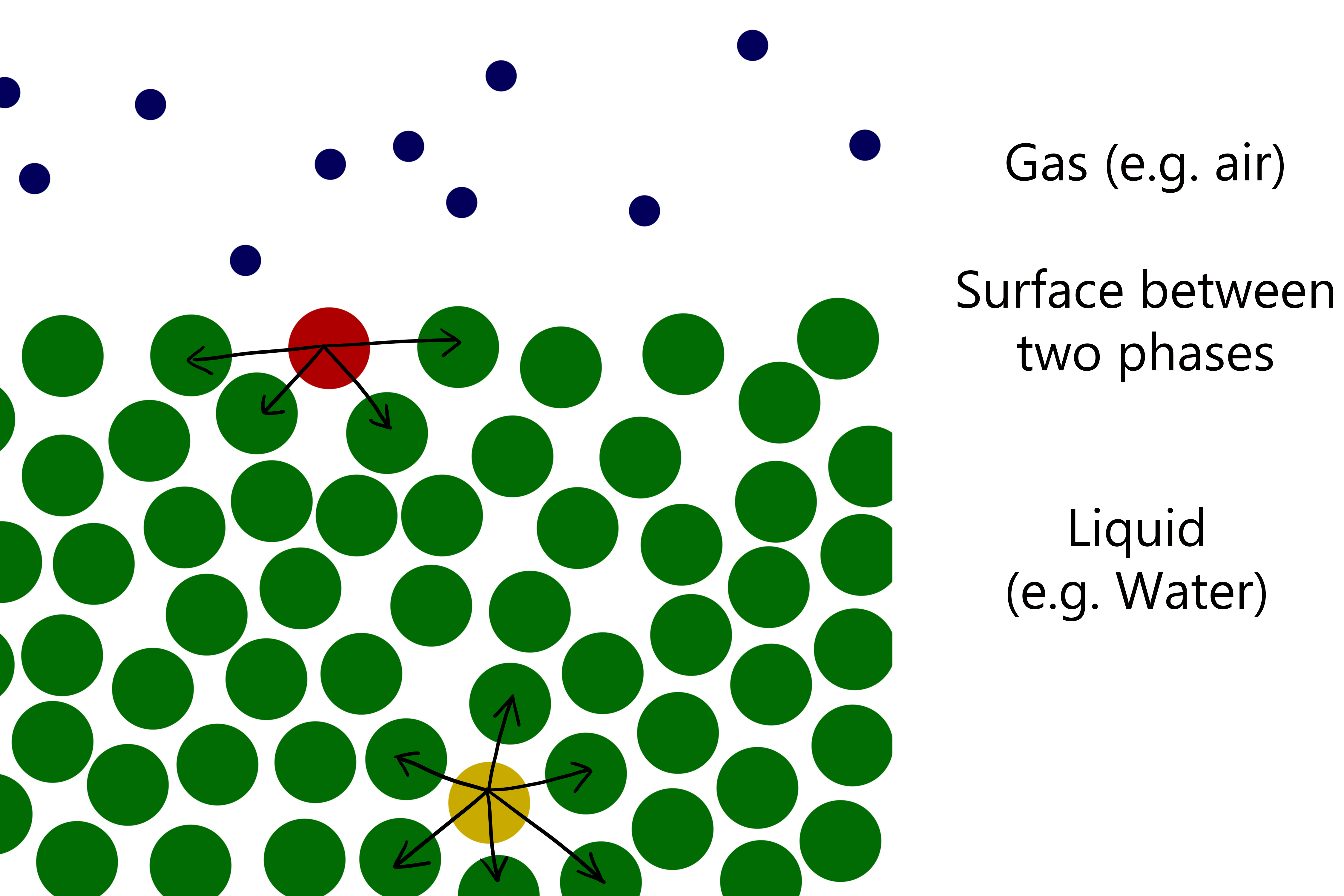
Surface tension
It requires energy to create a surface between two phases.
© University of the Highlands & Islands
Definition: |
Surface tension is the energy required per unit surface area to create a new interface isothermally and reversibly. (Energy required to overcome the opposing force and bring molecules to the surface). |
Unit: |
J/m2 |
Alternative definition: |
Surface tension is the force per unit length that acts perpendicularly to every line that can be drawn on the surface. |
Unit: |
N/m |
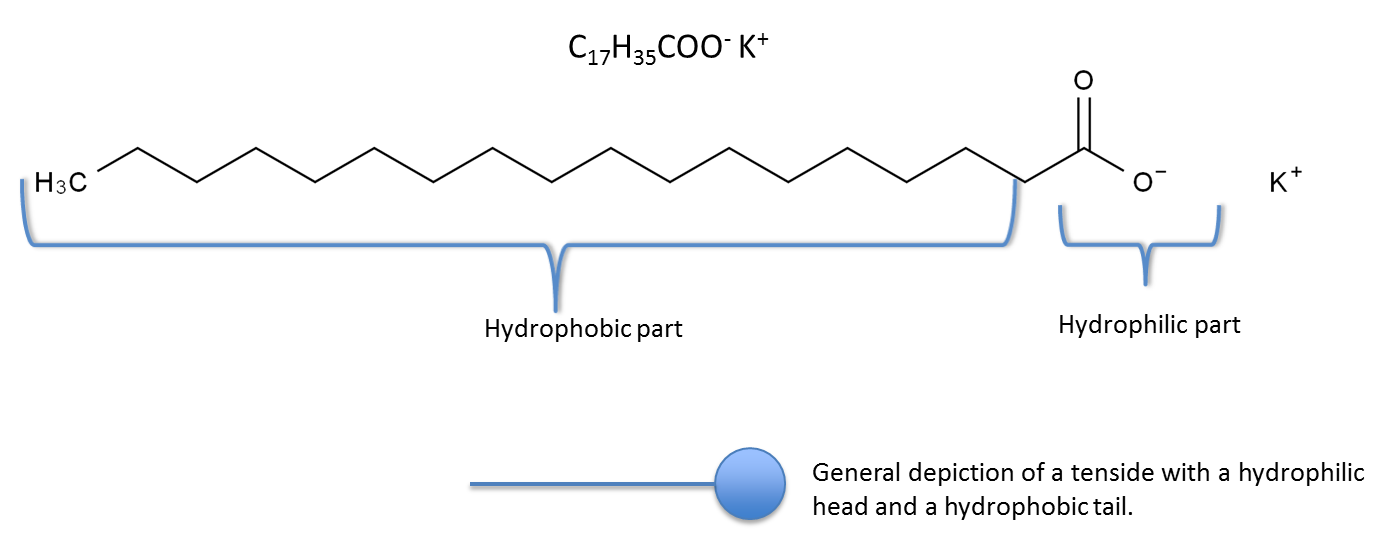
The tensides molecular structure causes them to have an affinity for interfaces which reduces the surface tension. Tensides consist of a hydrophilic (water loving) part and a hydrophobic (water hating) part. Potassium stearate is one example (used in washing soap).
© University of Borås
-
Project
This resource was developed as part of an Erasmus+ project, funded with support from the European Commission under grant agreement 2016-1-SE01-KA203-22064.
The project was a collaboration between:
This resource has been released under Creative Commons license CC-BY-SA 4.0.
Contact
If you would like more information on this resource please contact:
- Academic content – The University of Boras (www.hb.se)
- Technical resource development – The University of the Highlands and Islands Educational Development Unit - EDU (edu@uhi.ac.uk)
Disclaimer
Except where otherwise noted, this website is licensed under Creative Commons license CC-BY-SA 4.0. All images used under permission remain the copyright of the license holder.
PDF
Download a copy of this resource in PDF format.
You can also print individual pages by printing directly from the browser.
×







