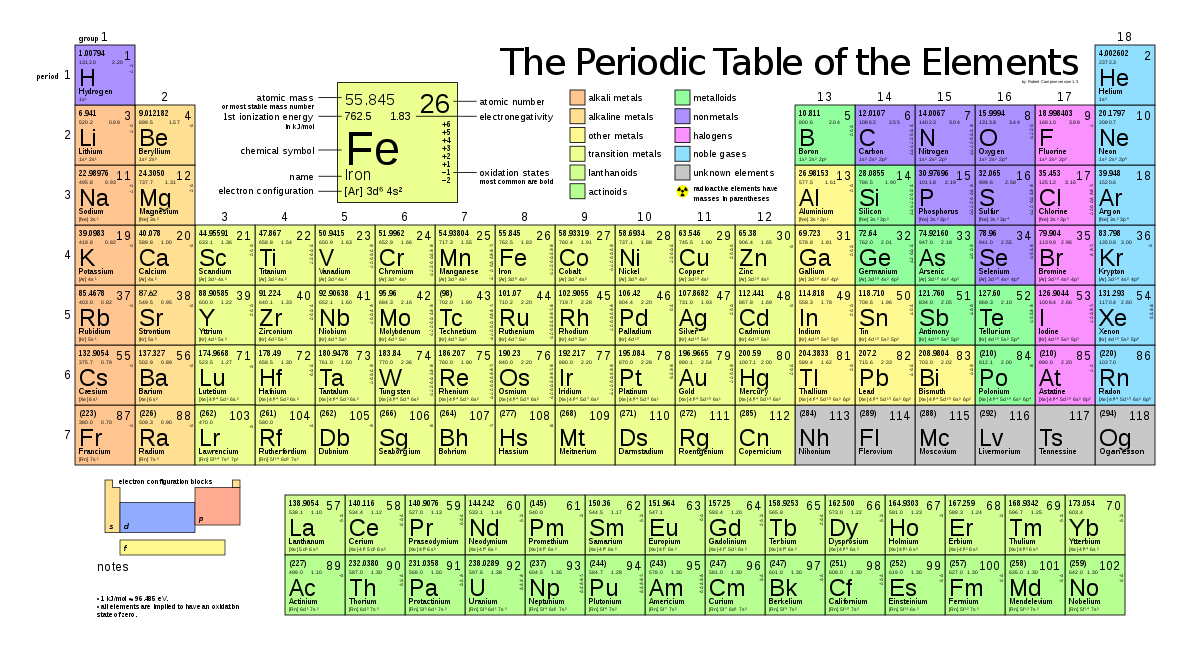
The periodic table of the elements
Period (row): how many electron shells are being utilized.
Group (column): how many electrons in the outer shell (He is exception).
The elements in period two:
- Inner (first) electron shell full
- Ne (Neon) has both the first and second shells full
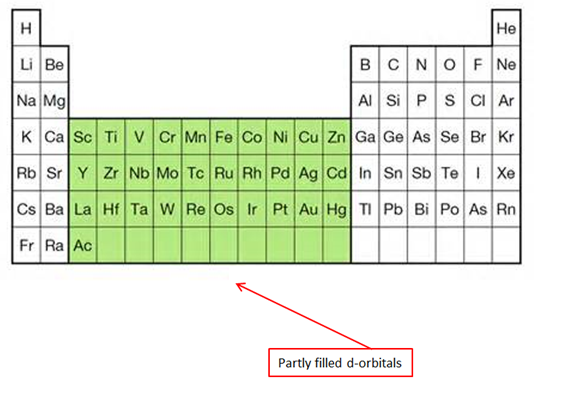
Shell three: Is first filled with 8 electrons (Ar) (room for 2*32=18 electrons)
- Before the remaining 10 are placed in shell three, shell four gets its first two electrons
© University of Borås