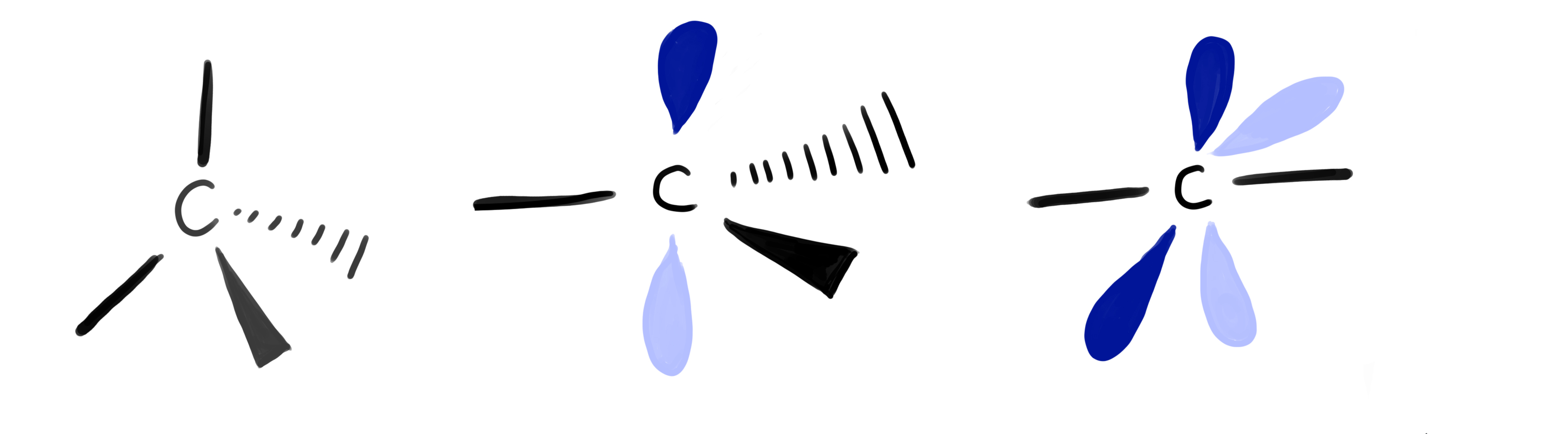
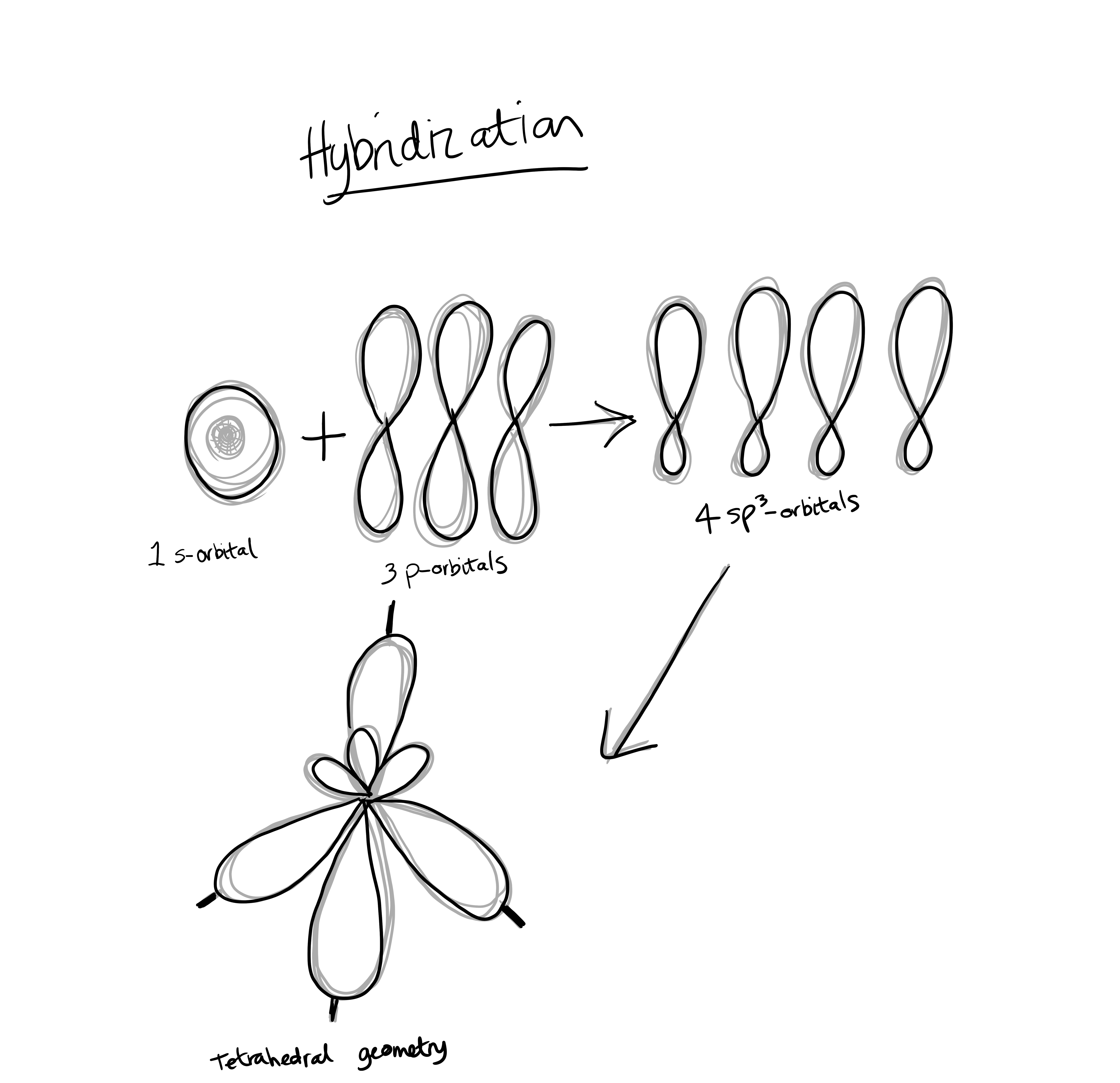
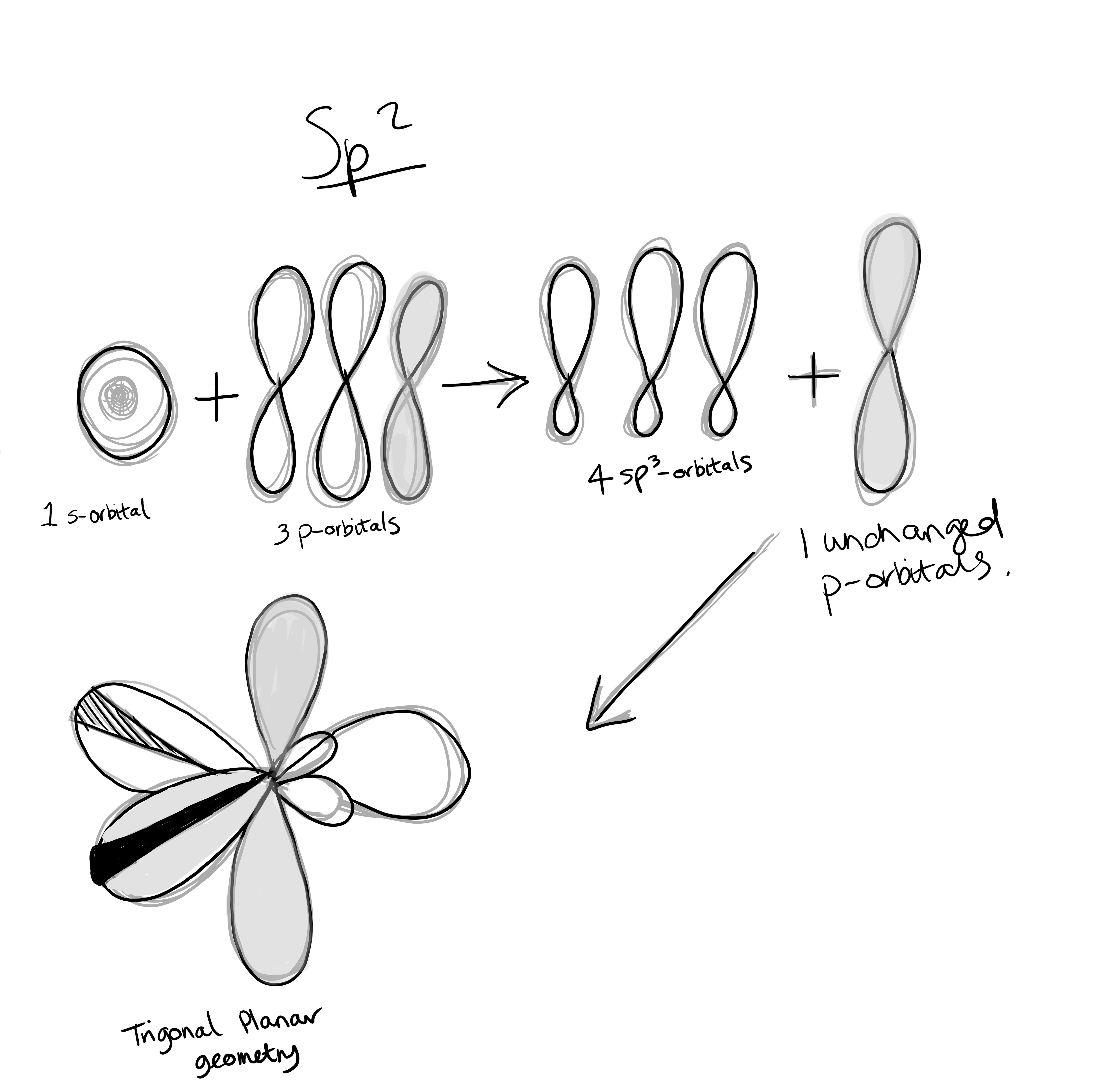
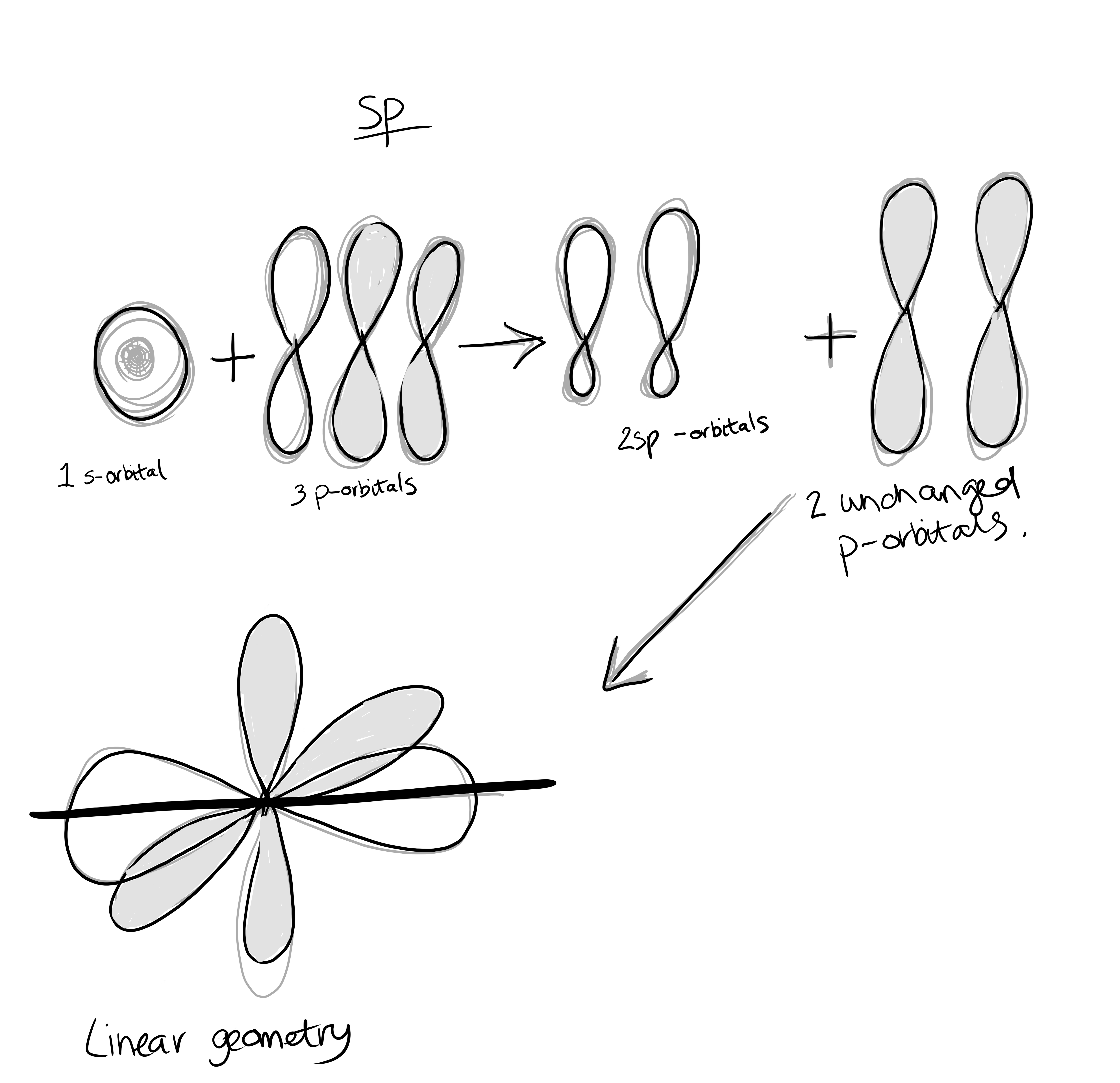
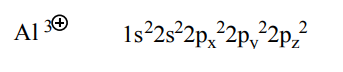Hybridization
Feedback
Q1 - When the elements lithium and chlorine react a salt is formed. Write the electron configuration of the ions in the salt formed.
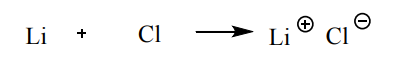
Electron structures:
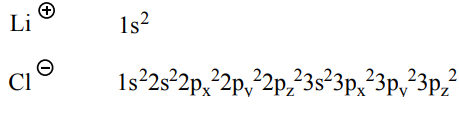
Q2 - In nature the element alumina, Al, exists as an ion. Write the electron configuration of this ion.