Properties of hydrocarbons
Feedback
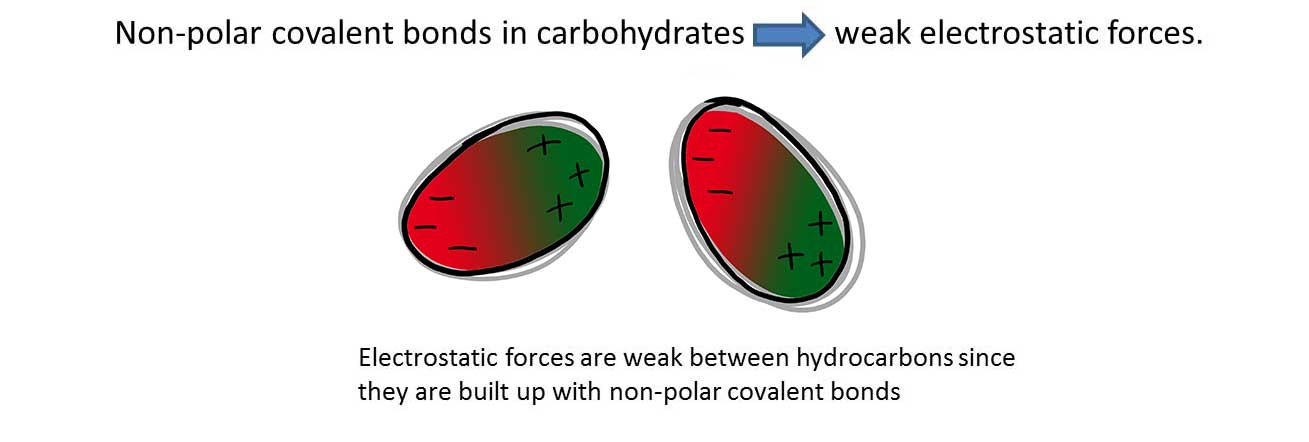
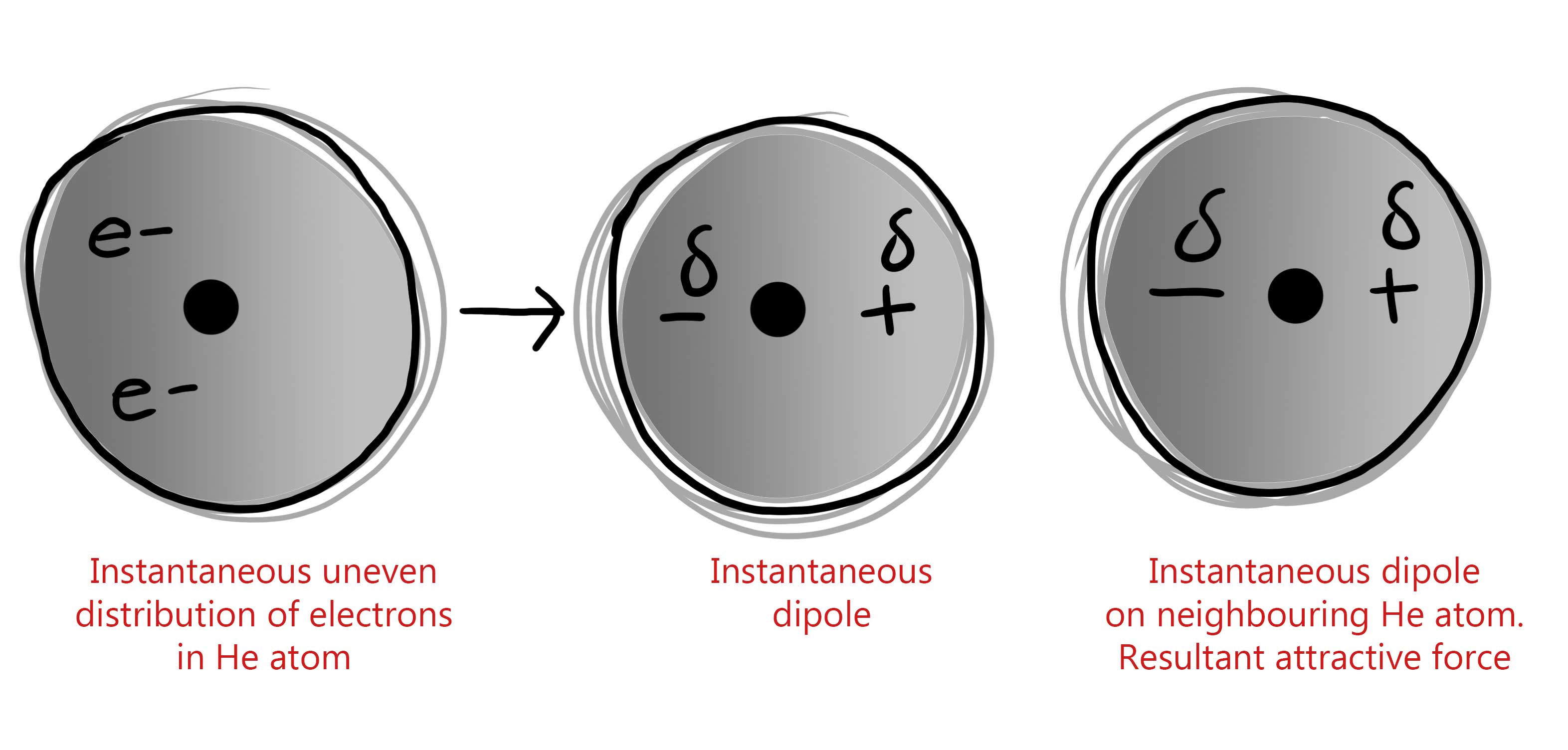
Q1 (a): Which is the dominating type of bond between two molecules of 1,2-dimethylcyclohexane?
A: Van der Waals bond
Q1 (b): Which is the dominating type of bond between two molecules of lactic acid?
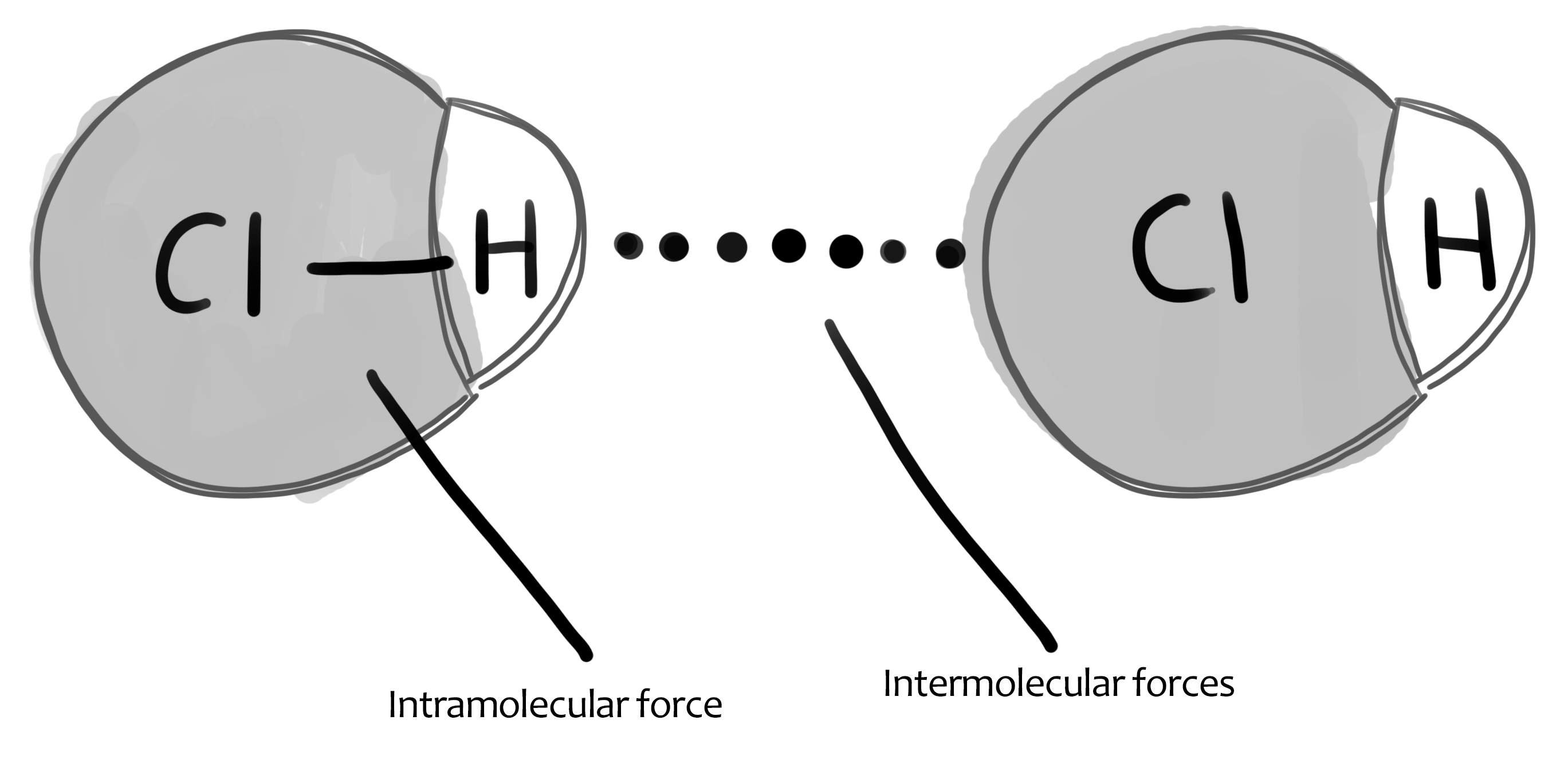
A: Hydrogen bond
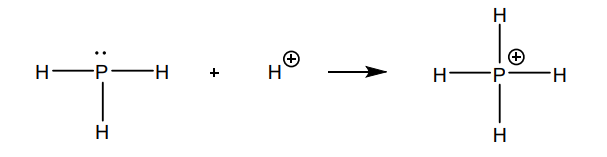
Q1 (c): Which different kinds of bonds are there between the atoms in the phosphonium ion?
A: Covalent bond and coordinative (covalent) bond
Feedback
Q2 (a): Which is the dominating type of bond between molecules of xylene?
A: Van der Waals bonds
Q2 (b): Which is the dominating type of bond between molecules of butanoic acid? ?
A: Hydrogen bonds
Q2 (c): Which is the dominating type of bond in solid CaO?
A: Ion bonds
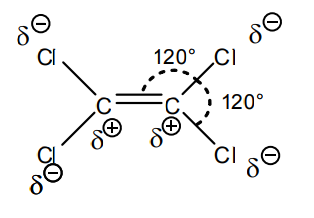
Q2 (d):Is the compound with the molecular formula C2Cl4 a dipole?
A: Tetrachloroethene is a planar compound (i.e. the three single bonds are in the same plane). All “outwards” groups have partial negative charge. Thus the compound will not orient itself in an exterior electric field. The compound is not a dipole
Feedback
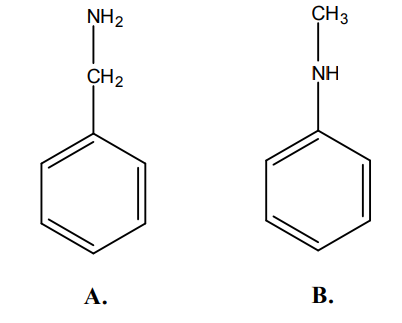
A is an alifatic amine, while B is an aromatic amine. Alifatic amines are more basic than aromatic ones. Thus A is more basic
×